Instructions
【Ingredients】 The main component of this product is trimethoprim, and its chemical name is 5-[(3,4,5trimethoxyphenyl)methyl]-2,4-pyrimidinediamine.
The structural formula is:
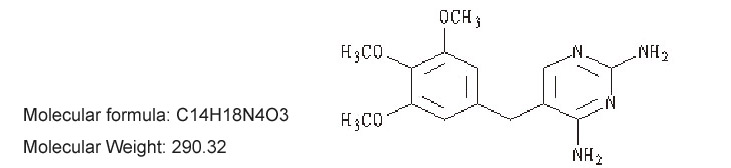
【Description】 This product is a white film.
【Indications】 This product can be used for acute simple lower urinary tract infections caused by bacteria such as Escherichia coli, Proteus mirabilis, Klebsiella pneumoniae and certain Enterobacter and Staphylococcus aureus First case. This product is not effective against Pseudomonas aeruginosa infection. At present, this product is rarely used alone, generally combined with sulfa drugs, such as sulfamethoxazole or sulfadiazine.
【Usage and Dosage】Treatment of acute simple urinary tract infection in adults usually used once a day 0.1g, once every 12 hours or once 0.2g, once a day, 7 to 10 days of treatment. Adult patients with impaired renal function need to reduce the application. Creatinine clearance rate >30m1/min (0.5m1/s) is still used in adults; creatinine clearance rate is 15~30ml/min (0.25~0.5nl/s), 50mg every 12 hours; creatinine clearance rate 15ml/ This product should not be used when min (0.25m1/s).
【Adverse Reaction】
1. Due to the interference of this product on folic acid metabolism, adverse reactions of the blood system may occur, and the reduction of white sclerotia, thrombocytopenia or methemoglobin anemia may occur. Generally, white blood cells and thrombocytopenia are mild, and timely withdrawal may be expected to be resumed, and folic acid preparation may also be added.
2. Allergic reactions: itching, rash, and even exudative polymorphous erythema.
3. Gastrointestinal reactions such as nausea, vomiting, diarrhea, and mild symptoms.
4. Occasionally, aseptic meningitis can occur, with headache, neck stiffness, nausea and other manifestations.
【Contraindications】
1. Newborns, premature infants are banned.
2. Severe liver and kidney disease, blood disease patients (such as leukopenia, thrombocytopenia, purpura, etc.) and those who are allergic to this product are prohibited.
【Precautions】
1. The following conditions should be used with caution: liver function damage; megaloblastic anemia due to folic acid deficiency or other blood system diseases; renal dysfunction.
2. During the medication, regular peripheral blood examination should be performed. Patients with long course of treatment, large doses, old age, malnutrition and taking anti-epileptic drugs are prone to folic acid deficiency. If the white blood cells or platelets in the surrounding blood have been significantly reduced, they need to stop. Use this product.
3. This product can be taken on an empty stomach. If you have gastrointestinal irritation, you can also take it with food.
4. If folic acid deficiency is caused by taking this product, folic acid preparation can be taken at the same time, the latter does not interfere with the antibacterial activity of the product, because the bacteria cannot utilize the synthesized folic acid. If there is a sign of myelosuppression, the product should be discontinued, and folic acid 3~6mg intramuscular injection should be given once a day for 3 days or as needed until the hematopoietic function returns to normal. For long-term and excessive use of this product, give high doses of folic acid and extend the course of treatment.
5. For patients with no urine, the half-life of this product can be extended from 10 hours to 20 to 50 hours. This product can be removed by hemodialysis, so the amount of maintenance should be replenished after dialysis; peritoneal dialysis has no effect on the removal of this product from blood.
6. If combined with sulfamethoxazole (SMZ), the dose ratio of both is preferably 1:5 (TMP: SMZ).
【Pregnant women and lactating women】
1. This product can cross the blood placenta barrier, although it has not been confirmed to have teratogenic effects in human applications, but because of its teratogenic effects on rats and rabbits, its mechanism of action is to interfere with folate metabolism. In the fetal circulation and amniotic fluid, the drug concentration is close to the maternal blood drug concentration, so the application of this product during pregnancy should be weighed against the pros and cons to decide whether to use the drug.
2. This product can be secreted into milk, its concentration is high, and the drug may interfere with the folate metabolism of breast-fed infants. Therefore, although the problem has not been confirmed in humans, this product must be weighed against the pros and cons after the application of the lactating mother. Medication.
【Children's medication】2 months to the baby should not apply this product
【older patients medication】elderly fools application of this product is prone to folic acid deficiency, the dosage should be reduced.
【Drug Interactions】
1. The opportunity for leukocyte and thrombocytopenia increases when the bone marrow inhibitor is combined with this product.
2. When the combination of dapsone and this product, the blood concentration of both can be increased, and the increase of the concentration of dapsone can increase the adverse reactions and increase the weight, especially the occurrence of methemoglobinemia.
3. This product should not be used simultaneously with anti-tumor drugs, 2,4-diaminopyrimidine drugs, and should not be applied between the treatments with other folic acid antagonists, because of poor bone marrow regeneration or megaloblastic anemia. Possible.
4. When combined with rifampicin, the product can be obviously cleared and the serum half-life is shortened.
5. Combined with cyclosporine can increase nephrotoxicity.
6. This product can interfere with the intrahepatic metabolism of phenytoin, increase the T1/2 of Christine sodium by 50%, and reduce its clearance by 30%.
7. In combination with procainamide, renal clearance of procainamide can be reduced, resulting in increased blood levels of procainamide and its metabolite, NAPA.
8. When combined with warfarin, it can inhibit the metabolism of the drug and enhance its anticoagulant effect.
【Drug overdose】 Excessive use of this product may cause nausea, vomiting, dizziness, headache, lethargy, confusion, bone marrow suppression. Overdose treatment: (1) gastric lavage. (2) Simultaneously give urine acidification drugs to promote the excretion of this product. (3) Supportive therapy. (4) Hemodialysis. Long-term use of this product can cause bone marrow suppression, resulting in the reduction of platelets, white blood cells and megaloblastic anemia. When there is a symptom of myelosuppression, the patient should immediately stop the drug and intramuscularly inject 5-15 mg of leucovorin per day until the hematopoietic function returns to normal.
【Pharmacology and Toxicology】 Trimethoprim (TMP) is a bacteriostatic agent, a lipophilic weak base, and its chemical structure is pyrimethamine. It has antibacterial activity against Escherichia coli, Klebsiella, S. variabilis, Salmonella and Shigella, and has no antibacterial effect against Streptococcus pneumoniae, Neisseria gonorrhoeae and Neisseria meningitidis. Obviously, it has no effect on Pseudomonas aeruginosa. The mechanism of action of this product is to interfere with the folate metabolism of bacteria. It mainly inhibits the activity of dihydrofolate reductase of bacteria, so that dihydrofolate cannot be reduced to tetrahydrofolate, and synthetic folic acid is a major component of nucleic acid biosynthesis, so this product blocks the synthesis of bacterial nucleic acids and proteins. And the combination of this product and the bacterial dihydrofolate reductase is 50,000 to 60,000 times more than the binding to the mammalian enzyme. The combination of wood and sulfa drugs can make the folic acid anabolism of bacteria to double block, and have a synergistic effect, which enhances the antibacterial activity of sulfa drugs, and can turn the bacteriostatic action into a bactericidal effect and reduce the production of drug-resistant strains.
【Pharmacokinetics】 This product is completely absorbed after oral administration, and can absorb more than 90% of the administered dose. The peak plasma concentration (Camx) arrives 1 to 4 hours after administration, and the peak plasma concentration after oral administration of 0.1 g is about It is 1 mg/L. After absorption, the product is widely distributed to tissues and body fluids, and the concentrations in kidney, liver, spleen, lung, muscle, bronchial secretions, saliva, vaginal secretions, prostate tissue and prostatic fluid all exceed the blood concentration. Wood products can cross the blood-cerebrospinal fluid barrier. When the meninges have no inflammation, the concentration of cerebrospinal fluid is 30% to 50% of the blood concentration, and the inflammation can reach 50% to 100%. TMP can also cross the blood placental barrier, and the drug concentration in the fetal circulation is close to the maternal blood concentration. The concentration of this product in milk is close to or higher than the blood concentration. The concentration of the drug in the aqueous humor is about 1/3 of the plasma concentration. The apparent volume of the product is 1.3~1.8L/kg; the protein binding rate is 30%-46%: the blood elimination half-life (t1/2β) is 8-10 hours, and the urine-free time can reach 20-50 hours. TMP is mainly filtered from the glomerulus, and the renal tubules are secreted and discharged. About 24% to 60% of the dose can be discharged in 24 hours, 80% to 90% of which is discharged as a drug, and the rest is excreted as a metabolite. The average urine concentration is 90~100mg/L, and the peak concentration in urine is about 200mg/L. In the acidic bed, the product is excreted from the urine, and the discharge in the alkaline urine is reduced. This product is excreted in small amounts from bile and feces (about 4% of the dose).
【Storage】Shading, sealed and stored.
【Package】Plastic bottle packaging: 100 pieces per bottle
【The shelf life】24 months.
【Executive Standard】Chinese Pharmacopoeia(2015) Volume Ⅱ.
【Approval No.】GYZZ H35020604
【Manufacturer】Fujian Pacific Pharmaceutical Co., Ltd.

