【Drug Name】:Celiprolol Hydrochloride Capsules
【Chinese Phonetics】:YAN SUAN SAI LI LUO ER JIAO NANG
【English Name】:Celiprolol Hydrochloride Capsules
Instructions
【Ingredients】The main component of this product is celiprolol hydrochloride, and the chemical name is 3-[3-acetyl-4-(3-Tetrabutylamino-2-hydroxy-propoxy)-phenyl]-1,1 -Diethyl urea hydrochloride, the chemical structural formula is:
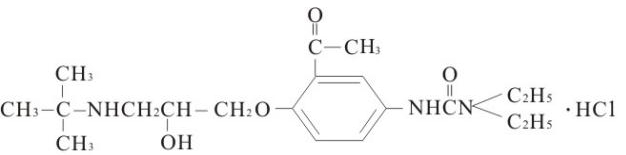
Molecular formula: C20H33N3O4·HCl
Molecular weight:415.96
【Description】 This product is a capsule, and the content is white granular powder or powder.
【Indications】mild to moderate hypertension.
【Strength】0.1g
【Usage and Dosage】Oral, 1-3 capsules (100~300mg) once a day, morning service or as directed by a doctor.
【Adverse Reactions】May have headache, dizziness, fatigue, drowsiness, lethargy and nausea. Generally, the reaction is mild, occasionally palpitations, tremors, usually no need to stop the drug, rare depression and allergic reactions. If there is a side effect related to β-blocker such as bronchospasm or rash, the drug should be discontinued.
【Contraindications】
1.Sinus bradycardia and severe bradycardia are contraindicated.
2. Right ventricular failure secondary to pulmonary hypertension is contraindicated.
3.Atrioventricular block above II degree is disabled.
4.Cardiogenic shock and severe heart failure are banned.
5.Antipsychotics that are taking augmentation of adrenaline activity and those who have discontinued such drugs for less than two weeks are banned.
6.Disabled during acute asthma attacks.
7.Severe kidney injury with creatinine clearance below 15 ml/min is contraindicated.
【Precautions】
1.Use with caution in patients with liver and kidney dysfunction.
2. When patients with angina pectoris and ischemic heart disease take this product for a long time, sudden withdrawal may cause angina pectoris and myocardial infarction. Therefore, for such patients, they should be gradually reduced under strict monitoring within 1-2 weeks until they are stopped.
3.Patients with congestive heart failure should be used with caution.
4.Patients with bronchospasm should be used with caution.
5.Use with caution in diabetic patients.
6.Patients with thyroid disease should be used with caution.
7.It is recommended to discontinue this product a few days before major surgery.
8.Athletes use with caution.
【Pregnant women and lactating women】This product can pass the placental barrier, so this product is not recommended.
【Child medication】This product is not recommended.
【Geriatric medication】It is not clear.
【Drug interactions】When combined with adrenergic neuron blockers such as reserpine, their effects may increase, sometimes with orthostatic hypotension and bradycardia or even dizziness and syncope. Both verapamil and beta-blockers can slow A-V transmission and inhibit myocardial contractility but have different mechanisms of action. When changing from Verapamil to this product or from verapamil, this product is recommended. The withdrawal period. If continuous dosing is required, ECG monitoring should be used initially. Patients with abnormal conduction should not be used in combination. Care should be taken when using a Class I antiarrhythmic drug such as propiamine. Food can reduce the bioavailability of this product. When combined with chlorthalidone and hydrochlorothiazide, it may also reduce the bioavailability of this product.
【Drug overdose】It is not clear.
【Pharmacological action】This product is a highly selective β-blocker that dilates blood vessels and lowers blood pressure by blocking the β1 receptor. This product binds highly selectively to the β1 receptor on the myocardial cell membrane, and its affinity is 20-30 times stronger than that of the bronchial and vascular smooth muscle β2 receptor. It can reduce heart rate and cardiac output during rest and exercise, reduce systolic blood pressure during exercise, and inhibit isoproterenol-induced tachycardia. For healthy people, this product does not reverse the vasodilating effect of isoproterenol mediated by the β2 receptor. This product has intrinsic sympathetic activity, does not increase respiratory resistance, expands peripheral blood vessels, and improves blood circulation. This product has no membrane stabilizing effect and does not inhibit myocardial contractility. It is less likely to cause sinus bradycardia than other β-blockers without endogenous sympathomimetic activity. This product can pass the placental barrier.
Non-clinical toxicology studies: The symptoms of animals in Kunming mice after oral administration or intravenous injection of celecolol were mainly sedation, startle, ataxia, skin flushing, and decreased activity. The above symptoms disappeared completely within 7 days. The LD50 of the drug for oral and intravenous injection was 1190.2 and 41.7 mg/kg.
【Pharmacokinetics】About 30% of this product can be absorbed after oral administration. The blood concentration reaches a high peak 2-4 hours after taking the drug. About 30% of the product is reversibly combined with plasma protein to eliminate the half-life of 3 -4 hours. This product can pass through the placental barrier, is not metabolized in the body, and is discharged in the original form, 10% of which is discharged from the urine and 85% from the feces.
【Storage】Shading, sealed and stored.
【Package】Aluminum plastic packaging, 12 tablets / plate × 1 plate / box.
【The shelf life】36 months.
【Executive Standards】National Drug Standard WS1-(X-465)-2003Z
【Approval No.】GYZZ H20020033
【Manufacturer】Fujian Pacific Pharmaceutical Co., Ltd.