Instructions
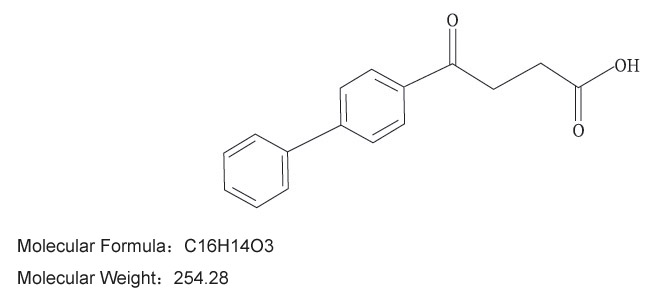
【Ingredients】The main component of this product is: Fenbufen (C16H14O3).
Chemical name: 3-(4-biphenylcarbonyl) propionic acid.
Chemical Structure:
【Description】This product is white or almost white tablets.
【Indications】For the treatment of rheumatoid arthritis, rheumatoid arthritis, osteoarthritis, spondyloarthropathy, gouty arthritis. It can also be used for toothache, post-operative pain and traumatic pain.
【Strength】0.3g
【Usage and Dosage】Oral.
The usual amount for adults: 0.6g a day, 1 time or 2 times. The total amount of adults per day does not exceed 1.0g.
Commonly used amount in children: not yet established
【Adverse Reaction】 The adverse reactions of this product are mainly gastrointestinal reactions, which are manifested as stomach pain, stomach burning sensation, nausea, and a few serious adverse reactions including gastric ulcer, blood stasis, and even perforation. Dizziness, rash, mild decrease in white blood cell count, and mild elevation of serum transferase are rare.
【Contraindications】
1. Patients who are known to be allergic to this product.
2. Patients who develop asthma, urticaria or allergic reactions after taking aspirin or other non-steroidal anti-inflammatory drugs.
3. Disable the treatment of perioperative pain in coronary artery bypass surgery (GABG).
4. Patients with a history of gastrointestinal bleeding or perforation after the application of non-steroidal anti-inflammatory drugs.
5. Patients with active peptic ulcer/bleeding, or who have had recurrent ulcers/bleeding in the past.
6. Patients with severe heart failure.
【Precautions】
1. Avoid combination with other non-steroidal anti-inflammatory drugs, including selective C0X-2 inhibitors.
2. According to the need to control symptoms, the use of the lowest effective dose within the shortest treatment time can minimize adverse reactions.
3. Adverse reactions to gastrointestinal bleeding, ulceration, and perforation may occur at any time during the course of treatment with all non-steroidal anti-inflammatory drugs, and the risk may be fatal. These adverse reactions may or may not be accompanied by warning symptoms, regardless of whether the patient has a history of gastrointestinal adverse reactions or a history of severe gastrointestinal events. Patients with a history of gastrointestinal disease (ulcerative colitis, Crohn's disease) should be careful to use non-steroidal anti-inflammatory drugs to avoid worsening the condition. When the patient takes this medicine to cause gastrointestinal bleeding or ulceration, the drug should be discontinued. Older patients use non-steroidal anti-inflammatory drugs to increase the frequency of adverse reactions, especially gastrointestinal bleeding and perforation, which may be fatal.
4. Clinical trials with multiple COXX-2 selective or non-selective NSAIDs for up to 3 years have shown that this product may cause an increased risk of severe cardiovascular thrombotic adverse events, myocardial infarction and stroke, which may be fatal. of. All NSAIDs, including C0X-2 selective or non-selective drugs, may have similar risks. Patients with cardiovascular or cardiovascular risk factors are at greater risk. Even if there are no previous cardiovascular symptoms, doctors and patients should be alert to the occurrence of such events. Patients should be informed of the signs and/or signs of severe cardiovascular safety and the steps that should be taken if they occur. Patients should be alert to symptoms and signs such as chest pain, shortness of breath, weakness, speech ambiguity, and should seek medical help immediately after any of the above symptoms or signs.
5. Like all non-steroidal anti-inflammatory drugs (NSAIDs), this product can cause new high blood pressure or exacerbate existing hypertension symptoms, any of which can lead to an increase in the incidence of cardiovascular events. The use of non-steroidal anti-inflammatory drugs (NSAIDs) in patients taking thiazides or myeloid diuretics may affect the efficacy of these drugs. Non-steroidal anti-inflammatory drugs (NSAIDs), including this product, should be used with caution in patients with hypertension. Blood pressure should be closely monitored during the start of treatment and throughout the treatment.
6. Patients with a history of hypertension and / or heart failure (such as fluid retention and edema) should be used with caution.
7. NSAIDs, including this product, may cause fatal, severe skin reactions such as exfoliative dermatitis, Stevens Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN). These serious events can occur without warning. Patients should be informed of the signs and symptoms of severe skin reactions. This product should be discontinued the first time a skin rash or other signs of an allergic reaction occur.
【Pregnant women and lactating women】is prohibited.
【Child medication】is prohibited.
【Geriatric Use】Elderly patients should pay attention to kidney toxicity due to decreased renal function.
【Drug Interactions】It is not clear.
【Drug overdose】It is not clear.
【Pharmacology and Toxicology】This product is a long-acting non-steroidal anti-inflammatory drug. It can inhibit the activity of epoxidase and reduce the synthesis of prostaglandins. Animal experiments show that the anti-inflammatory and analgesic effects of this product are weaker than that of indomethacin, but stronger than aspirin. The results of acute toxicity test: rat oral LDs50 was 200~720ng/kg, intraperitoneal injection of LD5 was 265-575mg/kg; mice oral LD50 was 795~1673mg/kg, and intraperitoneal injection LD50 was 506~811mg/kg.
【Pharmacokinetics】 80% of this product is absorbed about 2 hours after oral administration. The blood concentration of the active metabolite peaked at 6-8 hours. T1/2 is longer, about 7 hours, but 72 hours can measure the concentration in the blood. The plasma protein binding rate is 98% to 99%. 66% is excreted in the urine, 10% is discharged from the respiratory tract, and 10% is excreted in the feces.
【Storage】Shading, sealed and stored.
【Package】Aluminum-plastic packaging, 12 pieces per plate, 2 plates per box. Packed in plastic bottles, 100 tablets per bottle.
【The shelf life】36 months
【Executive Standard】Chinese Pharmacopoeia(2015) Volume Ⅱ.
【Approval No.】GYZZ H35020607
【Manufacturer】Fujian Pacific Pharmaceutical Co., Ltd.