Instructions
WARNING: After severe malaise, including tendonitis and tendon rupture, peripheral neuropathy, central nervous system effects and increased myasthenia gravis.
(1)The use of fluoroquinolone drugs (including the Norfloxacin lobe) has been reported to have both severe and potentially irreversible serious adverse effects (see 【Precautions】).
(2)Tendinitis and tendon rupture (see 【Precautions】).
(3)Peripheral neuropathy (see 【Precautions】).
(4)The effects of the central nervous system (see 【Precautions】).
(5)When these serious adverse reactions occur (see 【Precautions】), the norfloxacin capsule should be stopped immediately and fluoroquinolone drugs should be avoided.
(6)Fluoroquinolone drugs may aggravate the symptoms of myasthenia gravis in patients with myasthenia gravis. Patients with a history of myasthenia gravis should be avoided avoiding the use of norfloxacin capsules (see 【Precautions】).
(7)Serious adverse reactions have been reported due to the use of fluoroquinolone drugs (including norfloxacin capsules) (see 【Precautions】).
(8)For patients belonging to the following indications, norfloxacin capsules should be used in the absence of other medications: simple urinary tract infections (see【Indications】and 【Usage and Dosage】 )
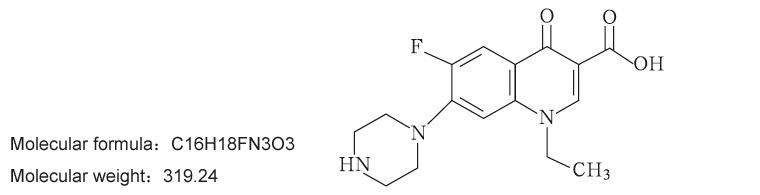
【Ingredients】The main ingredient of this product is norfloxacin.
Chemical name:1-ethyl-6-fluoro-1,4-dihydro-4 oxo-7-(1-piperazinyl)-3-quinolinecarboxylic acid.
Chemical Structure:
【Description】This product is a capsule, the content of which is white to light yellow granules or powder.
【Indications】Applicable to urinary tract infections caused by sensitive bacteria, gonorrhea, prostatitis, intestinal infections and typhoid and other Salmonella infections. Serious adverse reactions have been reported due to the use of fluoroquinolone drugs (including norfloxacin capsules), and for some patients, simple urinary tract infections are self-limiting and should be used without other medications. Star capsule.
【Strength】0.1g
【Usage and Dosage】
1. Acute simple lower urinary tract infection caused by Escherichia coli, Klebsiella pneumoniae and Proteus mirabilis 400mg once a day for 2 days.
2. The dose of simple urinary tract infection caused by other pathogens is the same as above, and the course of treatment is 7-10 days.
3. The dose of complicated urinary tract infection is the same as above, and the course of treatment is 10~21 days.
4. Simple gonococcal urethritis single 800~1200mg.
5. Acute and chronic prostatitis once 400mg, 2 times a day, the course of treatment is 28 days.
6. Intestinal infection once 300 ~ 400mg, 2 times a day, 5 to 7 days of treatment.
7. Salmonella typhimurium infection 800~1200mg a day, divided into 2~3 times, the course of treatment is 14~21 days.
【Adverse Reaction】
1. Gastrointestinal reactions are more common, which can be manifested as abdominal discomfort or pain, diarrhea, nausea or vomiting.
2. Central nervous system reactions can have dizziness, headache, lethargy or insomnia.
3. Allergic reactions rash, itchy skin, occasional exudative erythema and angioedema. A small number of patients have photosensitivity reactions.
4. Occasionally:
(1) seizures, mental disorders, irritability, disturbance of consciousness, hallucinations, tremors.
(2) Interstitial nephritis manifestations such as hematuria, fever, and rash.
(3) phlebitis.
(4) Crystalline urine, more common in high-dose applications.
(5) Joint pain.
5. A small number of patients can occur and the elevation of aminotransferase, blood urea nitrogen increased and peripheral blood leukocytes decreased, mostly mild, and transient.
6. Severe and other important adverse reactions:
(1) Disability and potentially irreversible severe adverseities should include post-creatitis and tendon rupture, peripheral neuropathy, and central nervous system effects.
(2) Tendon disease and tendon rupture.
(3) The QT interval is extended.
(4) Allergic reactions.
(5) Other serious and sometimes fatal reactions.
(6) The influence of the central nervous system.
(7) Clostridium difficile-associated diarrhea.
(8) Peripheral neuropathy.
(9) Interference with blood sugar.
(10) Light sensitivity / phototoxicity.
(11) Cardiovascular system: prolonged QT interval, torsade de pointive ventricular tachycardia, ventricular arrhythmia.
(12) Central nervous system: convulsions, toxic psychosis, tremors, agitation, anxiety, dizziness, confusion, hallucinations, delusions, depression, nightmares, insomnia, seizures, and very few cases can lead to suicidal thoughts or actions.
(13) Peripheral neuropathy: feeling disorganized, feeling dull, feeling of touch, pain, burning, tingling, numbness, weakness or light touch, pain, temperature, position and vibration abnormalities, polyneuritis.
(14) skeletal muscle system: joint pain, muscle pain, muscle weakness, tension hypertonic tendonitis, tendon rupture, worsening myasthenia gravis.
(15) Hypersensitivity: urticaria, itching and other serious skin reactions (such as toxic epidermal necrolysis, erythema multiforme), dyspnea, vascular nerves over edema (including tongue, throat, pharynx or facial edema / Swollen), cardiovascular collapse, hypotension, loss of consciousness, airway obstruction (including bronchospasm, shortness of breath and acute respiratory distress), hypersensitivity pneumonitis, anaphylactic shock.
(16) Hepatobiliary system: hepatitis, jaundice, acute liver necrosis or liver failure.
(17) urinary system: acutely overcome renal insufficiency or renal failure.
(18) Blood system: anemia, inclusion of hemolytic anemia and aplastic anemia, thrombocytopenia, including thrombotic thrombocytopenic purpura, leukopenia, neutropenia, pancytopenia, and/or other blood diseases.
(19) Others: fever, vasculitis, serum disease, Clostridium difficile-associated diarrhea, blood glucose disorder, photosensitivity/phototoxicity.
【Contraindications】Disabled for patients who are allergic to this product and fluoroquinolone.
【Precautions】
1. This product should be taken on an empty stomach, and drink 25Oml at the same time.
2. Since Escherichia coli is more common in norfloxacin-resistant patients, urine specimens should be kept before administration, and the drug sensitivity should be adjusted according to the results of bacterial susceptibility.
3. Crystallized urine can occur when the product is used in large doses or when the urine pH value is above 7. In order to avoid the occurrence of crystallized urine, it is advisable to drink more water and keep the urine output for more than 1200ml for 24 hours.
4. Patients with renal dysfunction need to adjust the dose according to renal function.
5. The application of fluoroquinolone drugs may occur after moderate and severe photoexgregation. Excessive exposure to sunlight should be avoided when applying this product. If photosensitive reaction occurs, stop the drug.
6. Glucose-6-phosphate dehydrogenase deficiency patients taking this product, very likely to have a hemolysis reaction.
7. Quinolones include this product can cause aggravation of myasthenia gravis, respiratory muscle weakness and life-threatening. The use of quinolones in patients with myasthenia gravis should include special products.
8. When liver function declines, such as severe (cirrhosis ascites) can reduce drug clearance, blood drug concentration is increased, liver and kidney function are all reduced, especially after weighing the pros and cons and applying dose adjustment.
9. Patients with original central nervous system diseases, such as those with epilepsy and epilepsy history, should avoid application. When there are indications, they should be carefully weighed against the pros and cons.
10. Disabling and potentially irreversible serious adverse effects, including tendinitis and tendon rupture, peripheral neuropathy, and effects of the central nervous system. The use of fluoroquinolone drugs has been reported to cause disabling and potentially irreversible serious adverse reactions in different organ systems of the same patient, usually including: tendonitis, tendon rupture, joint pain, myalgia, peripheral neuropathy and Central nervous system response (illusion, anxiety, depression, severe headache and confusion). These defects should occur after hours to weeks after the use of norfloxacin capsules. These adverse events have been reported in patients of any age without prior risk factors.
11. Tendons and tendon ruptures Fluoroquinolone drugs increase the risk of tendonitis and tendon rupture in patients of all ages. This adverse reaction most often occurs in the Achilles tendon, which may require surgical repair. There have also been reports of tendonitis and tendon rupture in the shoulders, hands, biceps, thumb and other tendon points. Tendinitis and tendon rupture can occur hours or days after the start of the use of norfloxacin capsules, or months after the end of treatment. Tendinitis and tendon rupture occur on the side. This risk is further increased in elderly patients over 60 years of age, patients taking corticosteroids, and patients undergoing kidney, heart or lung transplants. In addition to age and the use of corticosteroids, factors that independently increase the risk of tendon rupture include severe physical activity, renal failure, and previous tendon diseases such as rheumatoid arthritis. Tendinitis and tendon rupture also occur in patients who use fluoroquinolone drugs without the above risk factors. Tendon rupture can occur during or after treatment; it is also reported that tendon rupture occurs several months after the end of treatment. This product should be discontinued after the patient has muscle pain, swelling, inflammation or breakage.